After six months of planting, EDDS and EDTA amendments were added at 3 different rates 0,10,15 mM/kg. Ethylenediaminetetraacetic acid (EDTA) and S, S’ ethylenediamine disuccinate (EDDS) are common chelating agents. EDTA is one of the most effective chelating agents in increasing metal uptake by plants. One limitation is that EDTA has a low biodegradation rate and can persist long in the environment, increasing the risk of metal leaching. EDDS is a natural chelating agent that is able to biodegrade faster and therefore does not persist as long in the environment. The objective of this experiment was to evaluate the efficiency of EDDS as a chelating agent during lead phytoremediation (Datta, Sarkar, and Andra. 2007).
Soil and plant roots, shoots, and leach samples were collected 10 days after EDTA and EDDS treatment. Overall, vetiver grass showed no signs of toxicity to EDTA, EDDS, or in any of the lead concentrations. The lead concentration in the vetiver grass was from order of highest to smallest Millhopper, Immokalee, Tobosa, and Belle Glade. This is likely due to the soil’s acid pH and sandy nature. The higher pH and organic matter of the Belle Glade and Tobosa soils reduced the lead uptake in the vetiver root and shoot samples (Datta, Sarkar, and Andra. 2007).
The application of EDTA and EDDS at 10 and 15 mM/kg concentrations increased the lead uptake in the roots on the vetiver plant. Compared to the control group EDTA and EDDS accumulation 1 to 6 times lead uptake. An overall trend showed that lead uptake in the roots increased with increasing lead concentrations. The lead concentration in the roots was highest in the Millhopper and Immokalee and lowest in the Tobas and Belle Glade soil for all 3 lead treatments.
EDTA and EDDS were shown to increase the root to shoot translocation in the vetiver grass compared to the control. The shoot concentrations were highest in Millhopper and Immokalee and lowest in the Belle Glade and Tobosa soils. Overall, EDTA was shown to be more effective than EDDS.Overall, the chelating agents increased the concentration of soluble lead in all 3 treatments in all 4 soil types. Millhopper and Immokalee soils had the most soluble lead concentrations due to the acidic sandy nature of the soil. Due to the organic matter content and pH, the solubles of lead were significantly less in Belle Glade and Tobosa soils (Datta, Sarkar, and Syamsundar. 2007).
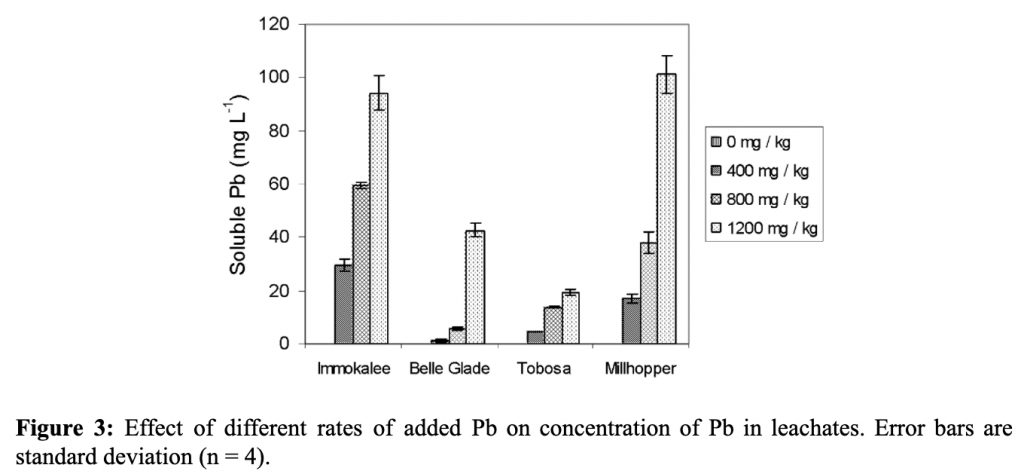
Leachates from the soil were collected and analyzed for soluble lead concentrations. Leaching was stimulated by overwatering. Compared to the control, a significant amount of lead was leached in the EDTA and EDDS treatments. As the lead concentration increased, the amount of lead leaching also increased. The physical-chemical properties of soil also affected the leaching of lead. Millhopper and Immokalee had a higher leaching lead than Tobasa and Belle Glade. This is because the clay content and higher pH made the lead less mobile ((Datta, Sarkar, and Syamsundar. 2007).
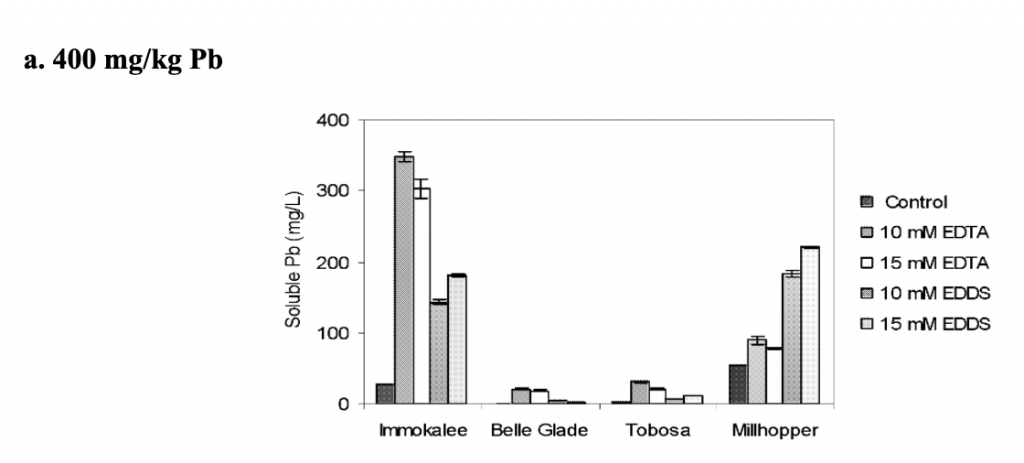
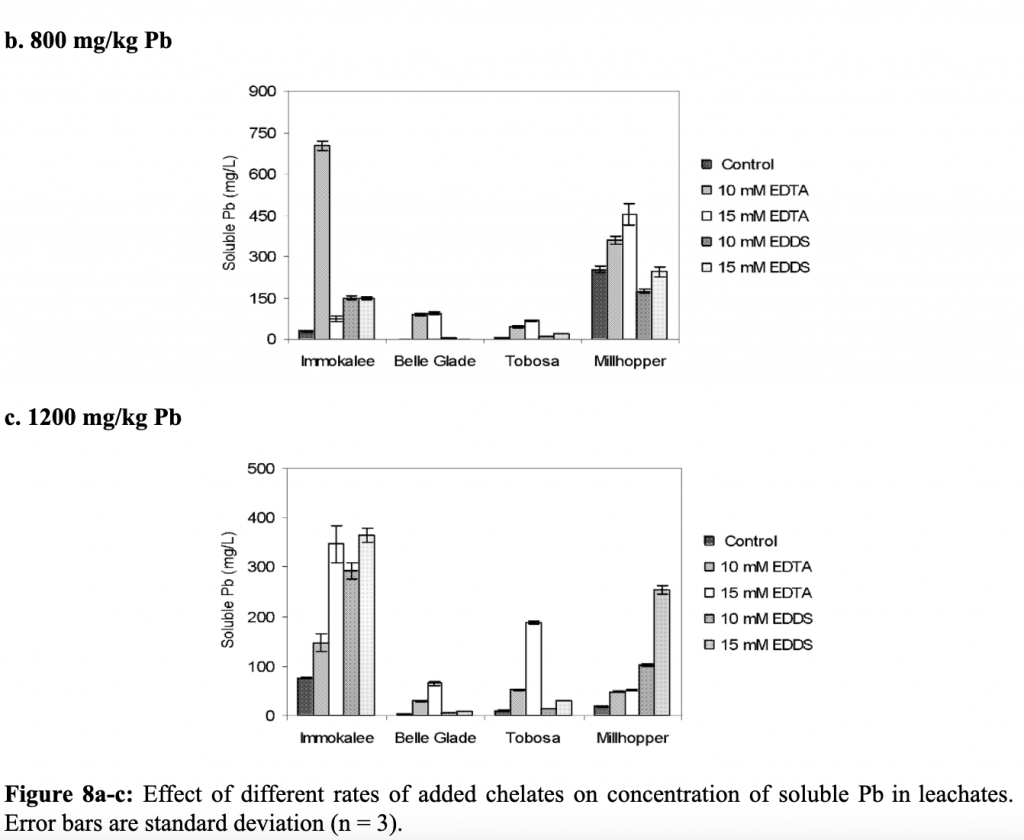
The overall effect of phytoremediation was analyzed at the end of the experiment. Total lead concentrations in the soil were determined. The amount of lead remaining in the soil was highest in Millhopper and Immokalee soils for all 3 amendments. The soils treated with EDTA and EDDS contained less lead than the control. Overall, EDDS was demonstrated to be a good chelating agent and proved safe phytoremediation of lead. EDDS increased root to shoot translation at concentrations of 15 mM/kg and showed similar results to EDTA (Datta, Sarkar, and Syamsundar. 2007).