An incubation greenhouse study was conducted to determine the efficiency of vetiver grass in lead phytoremediation. The objective of this study was to evaluate the efficiency of vetiver grass to remediate soils contaminated with lead-based paint. To determine the lead levels, soil pH, carbonate level, and clay levels of those soils to establish effective phytoremediation strategies. The soil was collected from 10 residential sites in San Antonio, Texas, and Baltimore, Maryland (Datta, Sarkar, and Andra. 2007).
The total lead values for San Antonio averaged 1697 ppm with a range between 36 – 4182 ppm. The average lead concentration for Baltimore was 697 ppm, ranging between 60 – 2165 ppm. The USEPA permissible limit is 400 ppm (Datta, Sarkar, and Andra. 2007).
Mehlich 3 extraction solutions were used to determine the amount of plant-available lead in the soil. The plant available lead in the San Antonio soil ranged between 9-46 %, while Baltimore ranged between 17-66 %. The soil pH for San Antonio was basic, ranging from 7.5 to 8.2. The pH for Baltimore was determined to be more acidic, with a pH ranging from 5.5 to 6.1. pH is a significant contributor to lead mobility; lead is more available for plant uptake with a lower soil pH. So it is more likely that Baltimore soil will have a higher metal uptake during phytoremediation (Datta, Sarkar, and Andra. 2007).
Organic matter and clay content also play a role in the success of phytoremediation and lead uptake. The carbonate content for San Antonio soils was 16 to 40%, while Baltimore soil ranged from 2 to 7%. The clay content for San Antonio is 3.5 to 60.5%, and Baltimore soil is between 1.8% and 4.9%. Given these results, Baltimore soil, with its acidic pH, low clay content, and low carbonate content, is likely that lead will be more available to plant uptake than San Antonio soil (Datta, Sarkar, and Andra. 2007).
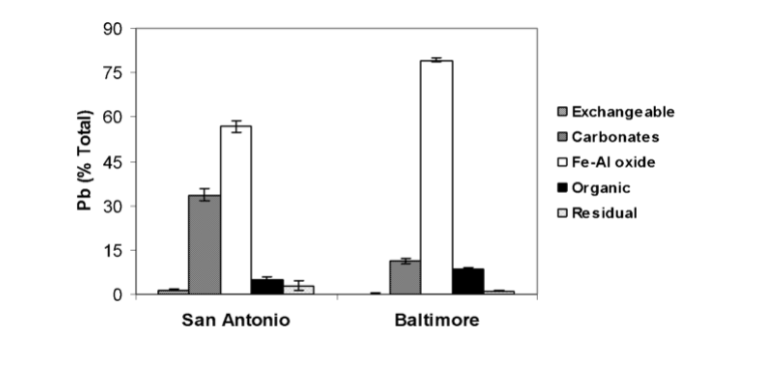
The geochemistry of lead in the soil was determined at time zero. For both soils, lead was found in Fe-Al oxide and carbonate fractions. Baltimore soil had 79% lead in the Fe-Al oxide phase, while San Antonio soil had 57%. Lead associated with carbonate ions was 33% in the San Antonio soil and 11% in the Baltimore soil. After determining the soil properties, vetiver grass was grown in PVC columns containing the soil. EDTA and EDDS chelating agents were added to test efficiency at 0,5,10 and 15 mM/kg rates. Plants were sampled and analyzed to metal uptake 10 days after added chelates (Datta, Sarkar, and Andra. 2007).
Plant root samples were analyzed for lead uptake. Compared to the controls, both EDTA and EDDS significantly increased the concentration of lead, with the highest at 15 mmol/kg. Both EDTA and EDDS increased the lead concentration for the shoot lead uptake. Lead concentration in the shoot increased from 22 to 476 mg/kg for Baltimore soils for EDTA and 22 to 493 mg/kg for EDDS for the 15 mmol/kg treatment. In the San Antonio soil, the lead concentration increased from 11 to 329 mg/kg for the EDTA and 240 mg/kg for the EDDS for the 15 mmol/kg treatment (Datta, Sarkar, and Andra. 2007).
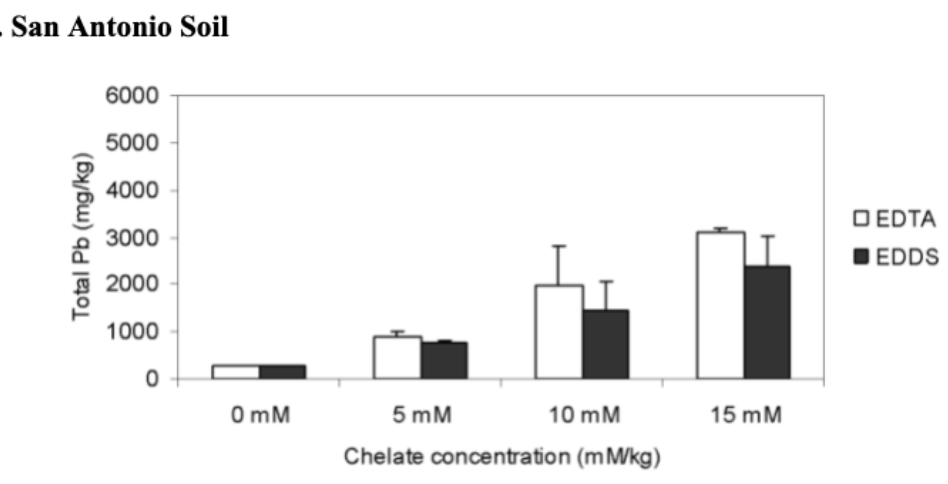
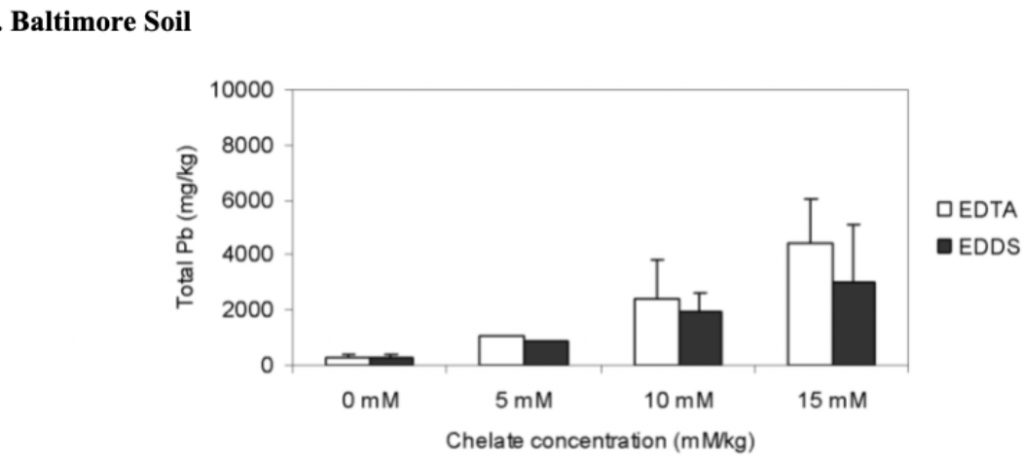
The effects of different rates of added chelates on the lead root uptake by vetiver grass in San Antonio and Baltimore soils
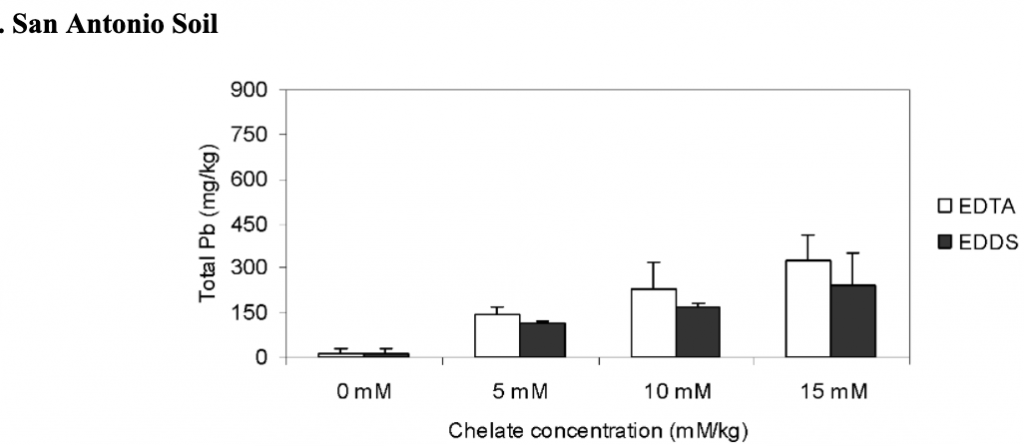
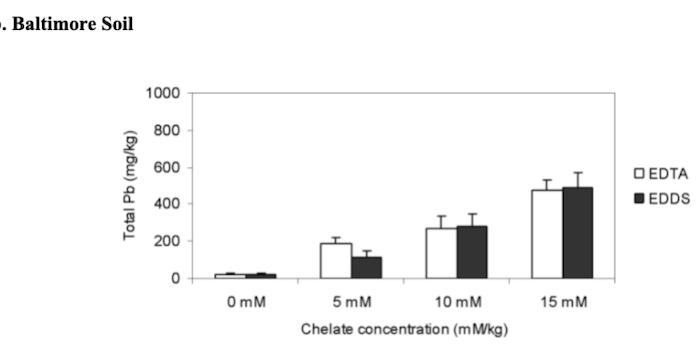
The effects of different rates of added chelates on the shoot lead accumulation by vetiver grass in San Antonio and Baltimore soils.
Leachates from the soil were collected and analyzed for soluble lead. The soluble lead increased with increasing lead concentrations. EDTA mobilized a significantly larger amount of lead compared to EDDS. The results indicated that the physical-chemical properties of soil had a strong influence on lead leaching from soils. Resulting in Baltimore soils having a significantly higher lead concentration than San Antonio (Datta, Sarkar, and Andra. 2007).
The geochemistry of lead in the soil was determined at the end of the experiment after adding EDTA and EDDS treatments. The percentage of lead associated with carbonate ions, organic fractions, and soluble + exchangeable increased with increasing chelating concentrations. In San Antonio soils, lead mainly was associated with Fe-Mn fractions with 50-79%, with a significant increase in the organic fraction from 16 to 30%. In Baltimore soils, the carbonate phase increased from 5% in control to 9% in chelated soils. The most considerable fraction of lead was the Fe-Mn oxide with 54-71% after adding chelating agents and the organic fraction with 17-38% (Datta, Sarkar, and Andra. 2007).
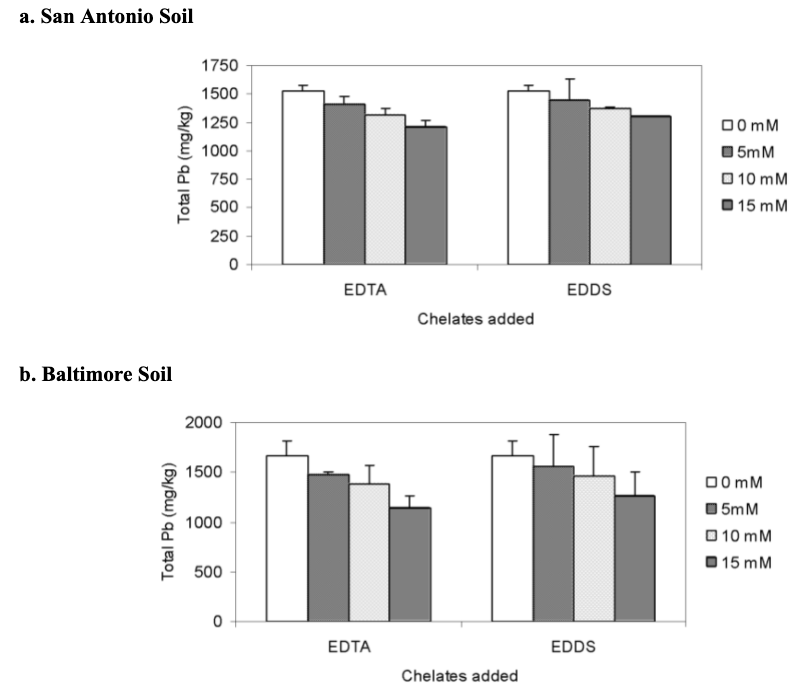
Total lead concentrations were analyzed at the end to determine phytoremediation and chelating agent efficiency. In general, soils treated with no EDTA and EDDS retained more lead. EDTA was more effective than EDDS. The concentration of total lead remaining in the soil was 15 mM/kg < 10 mM/kg < 5 mM/kg (Datta, Sarkar, and Andra. 2007).